Introduction
Silicone is a versatile material used in various industries such as kitchenware, medical devices, and electronics. One of the most remarkable characteristics of silicone is its high melting point and burn resistance, which allow it to remain stable in extreme heat. In this article, we’ll explore why silicone doesn’t melt or burn, explain its unique silicone melting point, and look into the various heat-resistant silicone products that make it an ideal choice for high-temperature environments.

What is Silicone?
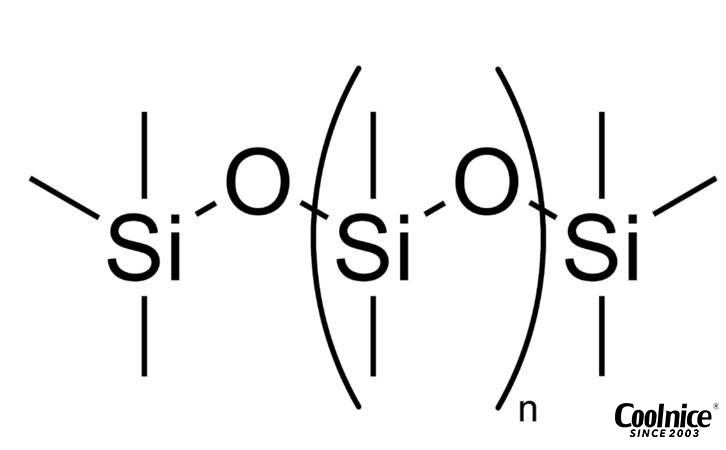
Silicone, also known as polysiloxane, is a polymer made up of repeating units of siloxane, a chain of alternating silicon and oxygen atoms, combined with carbon, hydrogen, and sometimes other elements. This unique molecular structure gives silicone its remarkable properties, including flexibility, durability, and high resistance to temperature extremes.
Silicone is used in various applications, including sealants, adhesives, lubricants, medical devices, cooking utensils, and electrical insulation. Some common forms of silicone include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk.
Silicone’s Unique Chemical Structure and Heat Resistance

Silicone’s ability to withstand high temperatures is due to its unique silicon-oxygen backbone. This structure forms a stable network that provides excellent thermal stability. Unlike plastics, which are made from carbon-based materials, silicone remains stable. The silicone melting point is typically much higher than most plastics, making it ideal for high-temperature applications like kitchenware, medical devices, and electronics.
Why Doesn’t Silicone Melt or Burn? The Silicone Melting Point Explained
The silicone melting point is one of the key reasons silicone doesn’t melt at high temperatures. Silicone is a thermosetting material, meaning that it doesn’t melt like thermoplastic materials. Instead, silicone decomposes at temperatures above 200°C to 450°C. Special formulations, such as fluorosilicone, can withstand even higher temperatures, making them suitable for extreme industrial and aerospace environments.

Unlike other materials, silicone doesn’t ignite easily. When exposed to high temperatures, silicone will not continue to burn once the source of fire is removed. Its burn resistance is one of the reasons silicone is used in high-temperature environments such as ovens and medical sterilization equipment.
Silicone’s Thermal Stability and Its Real-World Applications
Silicone’s high temperature resistance makes it an excellent material for many high-heat applications, including:
-
Kitchenware: Heat-resistant silicone products such as baking mats, spatulas, and oven mitts can withstand temperatures up to 230°C without losing their structural integrity.
-
Medical Devices: Silicone’s burn resistance and stability under heat make it a popular choice for medical devices, particularly those exposed to high temperatures during sterilization.
-
Electronics: Silicone is used in seals, gaskets, and insulation in electronic devices, where heat resistance is critical to preventing overheating and ensuring long-term reliability.

Silicone Melting Point vs Other High-Temperature Materials
When compared to other high-temperature materials, silicone excels in its ability to withstand heat without melting. While plastics and organic rubbers tend to melt and lose their shape under high temperatures, silicone remains stable, only starting to decompose at extreme temperatures above 200°C.
Silicone Burn Resistance and High-Temperature Stability
Unlike many other materials, silicone does not catch fire easily. Silicone burn resistance is attributed to its silicon-oxygen backbone, which is highly stable even at high temperatures. When exposed to fire, silicone will not continue to burn after the flame is removed. It will decompose into silicon dioxide (SiO₂), which is non-combustible and self-extinguishing.
Special silicone formulations can withstand even higher temperatures, such as fluorosilicone, which can endure temperatures up to 350°C and is ideal for industrial and aerospace applications.
FAQs about Silicone’s Heat Resistance
-
Q1: Why doesn’t silicone melt or burn?
Silicone’s unique chemical structure, especially the silicon-oxygen bonds, makes it highly resistant to heat. Unlike plastics, silicone does not have a traditional melting point. Instead, it decomposes at high temperatures. -
Q2: What is the maximum temperature silicone can withstand?
Silicone can typically withstand temperatures between −40°C and 230°C, and certain formulations can endure up to 350°C. -
Q3: Can silicone be used in ovens or microwaves?
Yes, food-grade silicone is safe for use in ovens and microwaves, withstanding temperatures up to 230°C without deforming. -
Q4: Why does silicone burn but not melt?
When exposed to high heat, silicone decomposes into silicon dioxide (SiO₂), which is self-extinguishing. It doesn’t melt because of its cross-linked structure. -
Q5: How does silicone decompose?
When exposed to high temperatures, silicone decomposes into silicon dioxide, a non-combustible substance that doesn’t support further combustion.
Conclusion
Silicone is an extraordinary material with unique heat-resistant and burn-resistant properties. Understanding why silicone doesn’t melt or burn can help manufacturers and engineers make informed decisions when selecting materials for high-temperature environments. Silicone’s high melting point and silicone burn resistance make it an ideal choice for kitchenware, medical devices, and industrial applications.
Call to Action (CTA)
If you need custom heat-resistant silicone products or further information on silicone’s heat resistance, contact us today for a consultation.